Find H for the Solution of 1.00g Naoh in Water
Calorimetry -qsystem = qsurroundings Using DT, which can be measured, and the heat capacity of the solvent in the coffee cup, the heat lost by the system can be calculated. Since the pressure is constant, this is equal to the enthalpy change for the process. NaOH(s) Step 1: Define the system and surroundings. Write your answer in the space below, then click on the check button. | |
| Now compare your answer with the one below. Please enter your answer in the space at left. | |
| As in most thermochemical equations, the reactants and products, the NaOH(s) and NaOH(aq) in this case, are the best choice for the system. Because of the thermal insulation provided by the styrofoam in the calorimeter, we can confine our definition of the surroundings to the water contained in the calorimeter. | |
| Step 2: Identify and assign signs to all the kinds of energy or work entering or leaving the system. Write your answer in the space below, then click on the check button. | |
| Now compare your answer with the one below. Please enter your answer in the space at left. | |
| Since the temperature of the water increased, heat must have been transferred from the system to the surroundings. This heat therefore has a negative sign. | |
| Step 3: Predict the units your answer should have. Write your answer in the space below, then click on the check button. | |
| Now compare your answer with the one below. Please enter your answer in the space at left. | |
| The question is asking for an enthalpy, so the answer should be in joules or kilojoules. | |
| Step 4: Predict the approximate size of your answer. Write your answer in the space below, then click on the check button. | |
| Now compare your answer with the one below. Please enter your answer in the space at left. | |
| The heat capacity of water is about 4 J/gºC, so the heat transferred should be about 100*4*3 = 1200 J. Since a thermochemical equation relates the heat transferred to moles of reactants or products, this should be expressed as a ratio to the amount of NaOH dissolved, or 1200 J/1 g NaOH * 40 g/mol = 48000 J or 48 kJ. | |
| How much heat is gained by the water in the calorimeter? Click on the correct answer below. | |
| 1113 J 11,573 J 11.3 J | |
| Correct! Since -qsystem = qsurroundings, this is also the heat lost by the system. Remember, DT = Tfinal - Tinitial. Remember to include the mass of the water in the calorimeter in your calculation. | |
| What is the enthalpy change per mole of NaOH(s)? This will be the DH for the thermochemical equation. Write your answer in the space below and click on the Review Answers button. | |
| | |
| Correct! Remember to round your answer to the correct number of significant figures. Remember, the system is losing energy, so the sign must be negative. Remember, the DH must be expressed as a ratio to the number of moles of reactants or products in the thermochemical equation. That is incorrect, please try again. The correct answer is -44.5 kJ. | |
Find H for the Solution of 1.00g Naoh in Water
Source: https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/thermodynamics/chemical/chemical2.htm
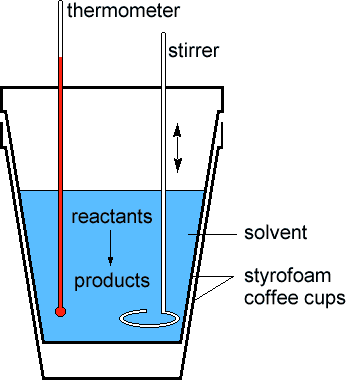 Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter
Scientists measure the change in thermodynamic quantities in thermochemical equations using a device known as a calorimeter